Managing Postoperative Nausea and Vomiting
The Prevalence of PONV
Postoperative nausea and vomiting are 2 of the most common adverse events
following surgery, with an estimated incidence of 30% in the general
surgical population and up to
Ondansetron, one of the most commonly used antiemetics, has a relatively short half-life (3 to 6 hours). Even when treated with ondansetron or other antiemetics, more than 30% of patients still experience PONV within the first 48 hours after surgery—well after many outpatient surgery patients have been discharged and no longer have access to fast-onset IV antiemetics or direct care.2,3
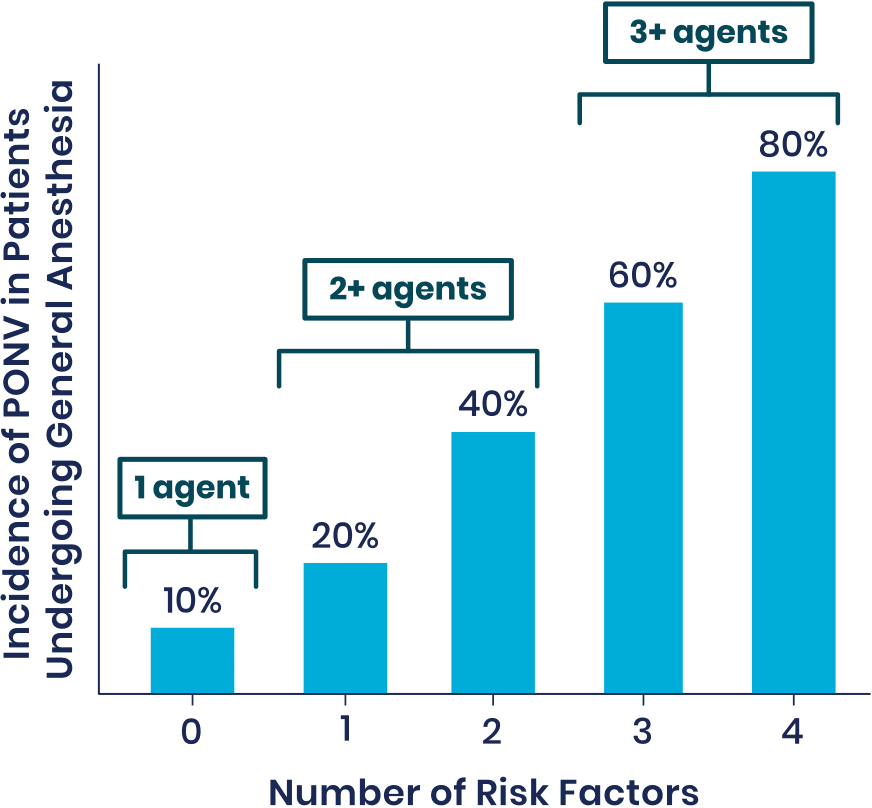
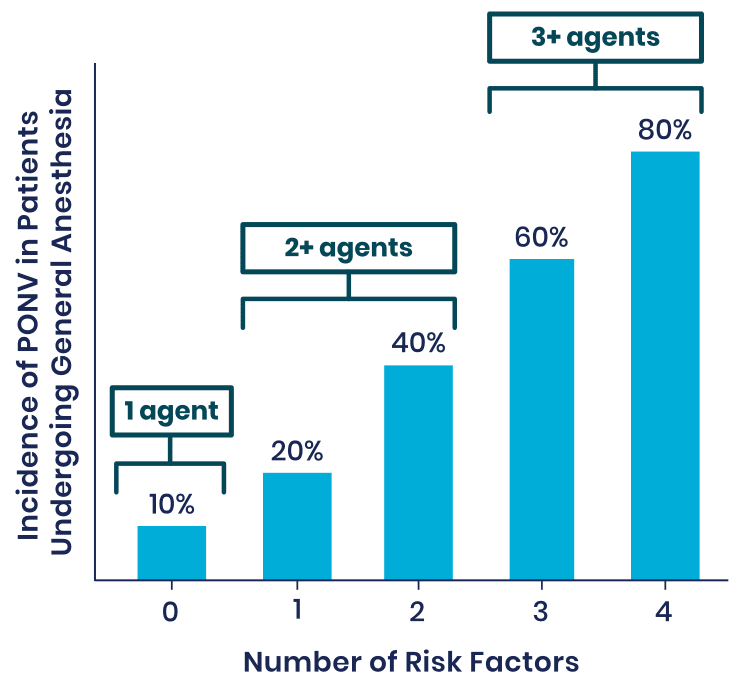
Risk Factors for PONV1
Patients at Moderate-to-High Risk of PONV Exhibit the Following Traits:
- Females
- Non-smokers
- Patients with a history of PONV or motion sickness
- Patients who are treated with opioids
PONV is a Significant Burden to Patients and Facilities
PONV is a major cause of patient dissatisfaction after surgery, with patients ranking vomiting as the most undesirable outcome when asked about postsurgical complications4
Poorly managed PONV affects overall length of hospital stay and may require unanticipated hospital readmission, which can lead to increased cost to the patient and to the healthcare system5-7
Clinical complications of persistent retching or vomiting can include7,8:
- Aspiration
- Tension on suture lines
- Development of hematomas beneath surgical flaps
- Electrolyte abnormalities
- Dehydration
Enhanced recovery after surgery (ERAS) protocols include evidence-based practices to improve patient outcomes, including9:
- Reduced opioid consumption
- Decreased PONV
- Decreased length of stay
References: 1. Gan TJ, Belani KG,
Bergese S, et al. Fourth consensus guidelines for the management of postoperative
nausea and vomiting. Anesth Analg. 2020;131(2):411-448. doi:10.1213/
ane.0000000000004833.
Important Safety Information!
Contraindications
APONVIE is contraindicated in patients with a history of hypersensitivity to aprepitant or any component of the product, and in patients taking pimozide. Increased pimozide levels may cause serious or life-threatening reactions, such as QT prolongation.
Warnings and Precautions
Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis, during or soon after administration of aprepitant have occurred. Symptoms including dyspnea, eye swelling, flushing, pruritus, and wheezing have been reported. Monitor patients during and after administration. If hypersensitivity reactions occur, administer appropriate medical therapy. Do not administer APONVIE in patients who experienced these symptoms with previous use of aprepitant.
Clinically Significant CYP3A4 Drug Interactions: Aprepitant is a substrate, weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Use of pimozide, a CYP3A4 substrate, with APONVIE is contraindicated. Use of APONVIE with strong CYP3A4 inhibitors (eg, ketoconazole) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to APONVIE. Use of APONVIE with strong CYP3A4 inducers (eg, rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of APONVIE.
Decrease in INR with Concomitant Warfarin: Use of aprepitant with
warfarin, a CYP2C9 substrate, may result in a clinically significant
decrease in the International Normalized Ratio (INR) of prothrombin time.
Monitor the INR in patients on chronic warfarin therapy in the 2-week period
particularly at 7 to
Risk of Reduced Efficacy of Hormonal Contraceptives: The efficacy of
hormonal contraceptives may be reduced for
Use in Specific Populations
Avoid use of APONVIE in pregnant women as alcohol is an inactive ingredient in APONVIE. There is no safe level of alcohol exposure in pregnancy.
Adverse Reactions
Most common adverse reactions (incidence ≥3%) for APONVIE are constipation, fatigue, and headache and for oral aprepitant are constipation and hypotension.
Report side effects to Heron at
Indication
APONVIE is a substance P/neurokinin-1 (NK1) receptor antagonist, indicated for the prevention of postoperative nausea and vomiting (PONV) in adults.
Limitations of Use: APONVIE has not been studied for treatment of established nausea and vomiting.
Please see full Prescribing Information.
Connect with Heron!
How could you incorporate APONVIE as the foundation of your institution's PONV management strategy? Connect with us to find out.
Fields marked with an asterisk (*) are required.
To report an adverse event or product complaint:
844-HERON11 (844-437-6611)
MedInfo@HeronTx.com
For general information:
Investors and Media:
For all other inquiries, please visit the Heron Therapeutics corporate website.
Go to Heron Website