Phase 3 Clinical Studies
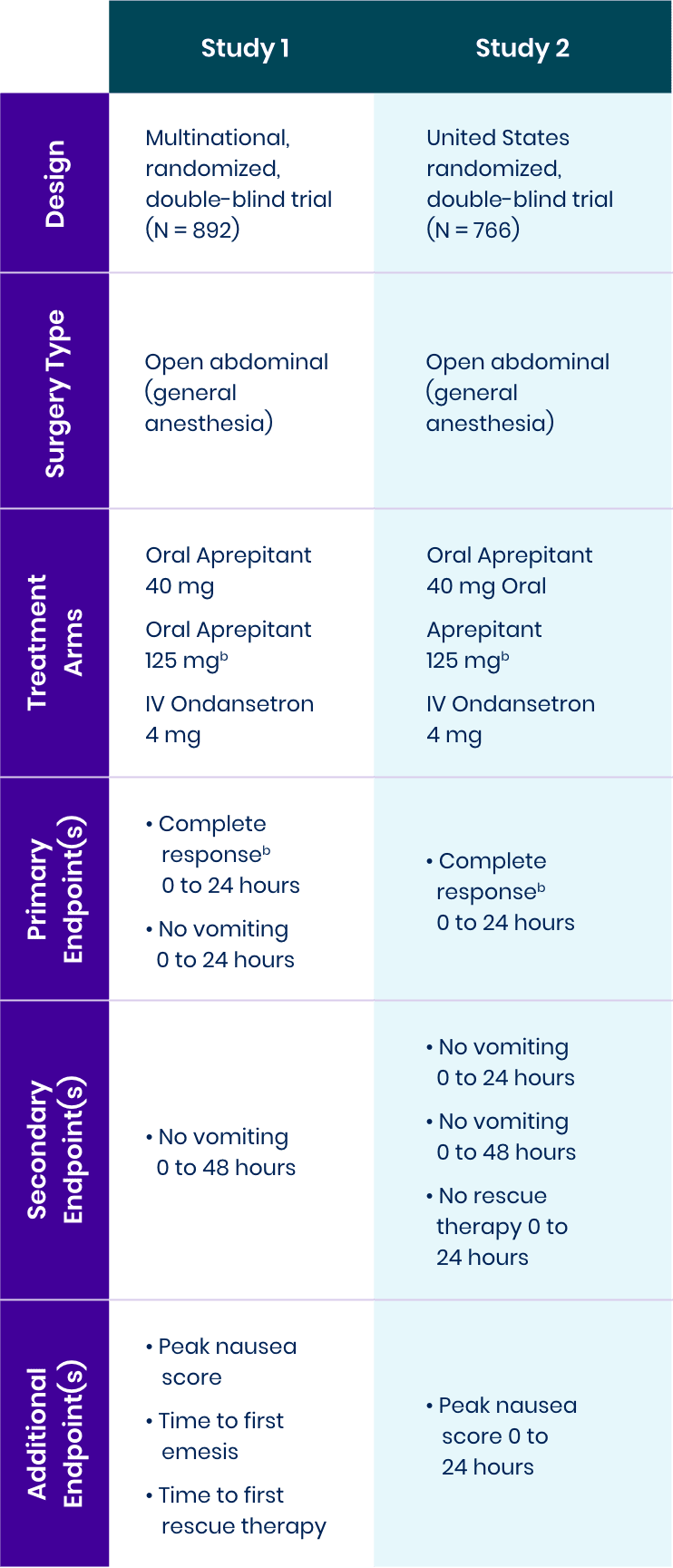
Study Design1-3
Two Phase 3, multicenter, randomized, double-blind, active-controlled trials in patients receiving general anesthesia for open abdominal surgery were conducted to test the efficacy of NK1 antagonist oral aprepitant versus standard-of-care IV ondansetron.
aThere were no
clinically meaningful differences between the
bComplete response was defined as no vomiting and no use of rescue therapy.
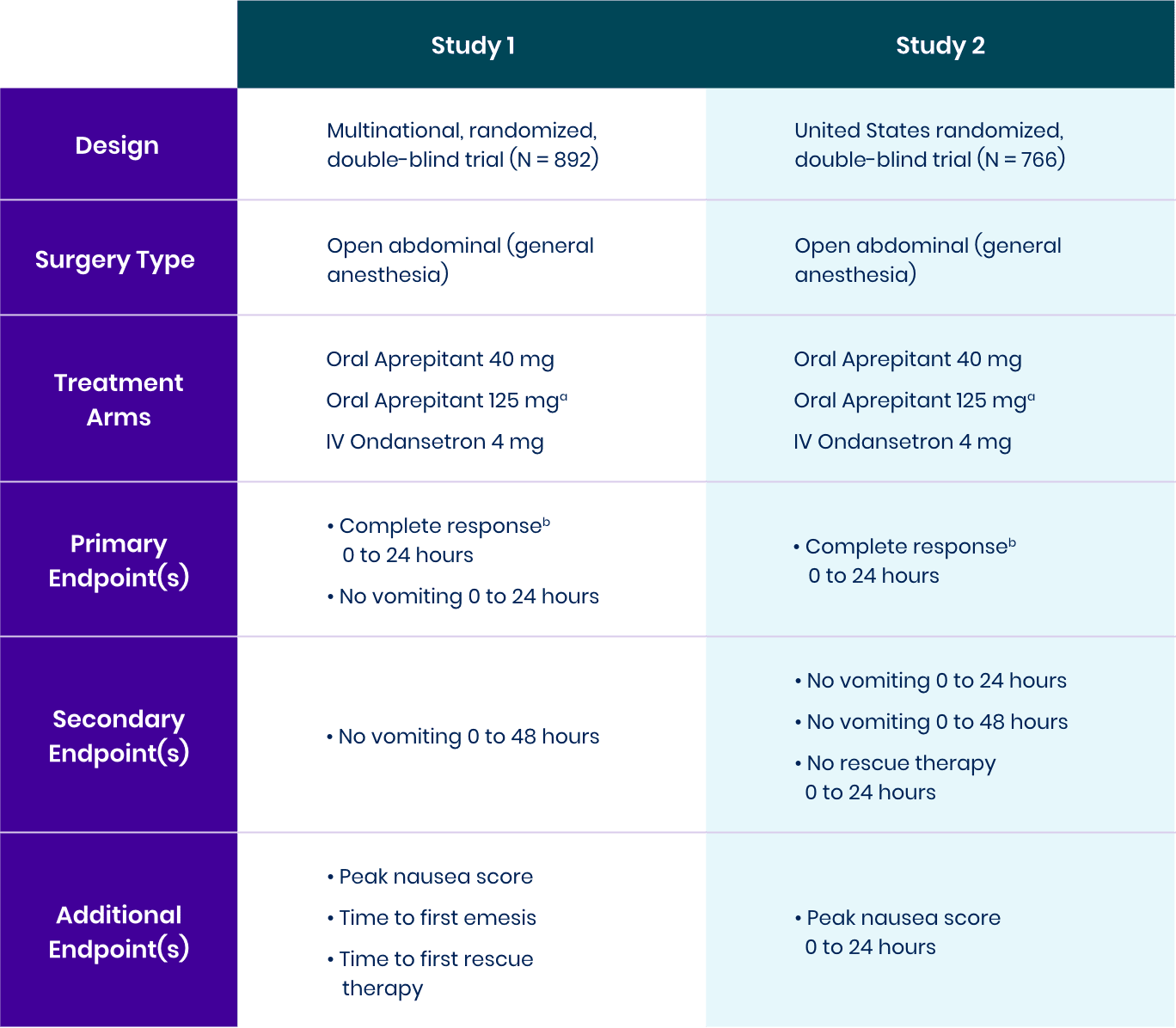
View bioequivalence studyComplete Response: No Vomiting and No Use of Rescue Therapy1-3
Study 1 Co-Primary Endpoint
% patients with complete response,
Aprepitant demonstrated superiority to IV ondansetron (1-tail 97.5% CI of OR: LB = 1.02a).
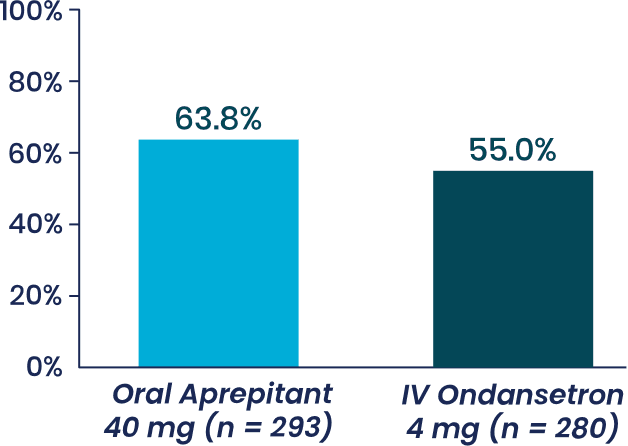
Study 2 Primary Endpoint
% patients with complete response,
Aprepitant did not demonstrate
superiority to IV ondansetron (
Superior Vomiting Prevention Through 24 hours
1-3
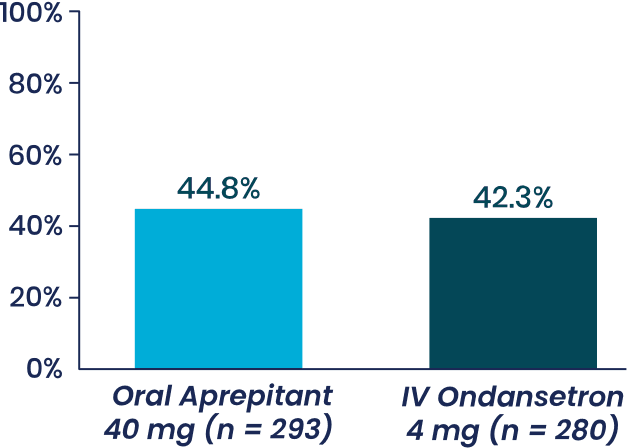
Study 1 Co-Primary Endpoint
% patients with with no vomiting,
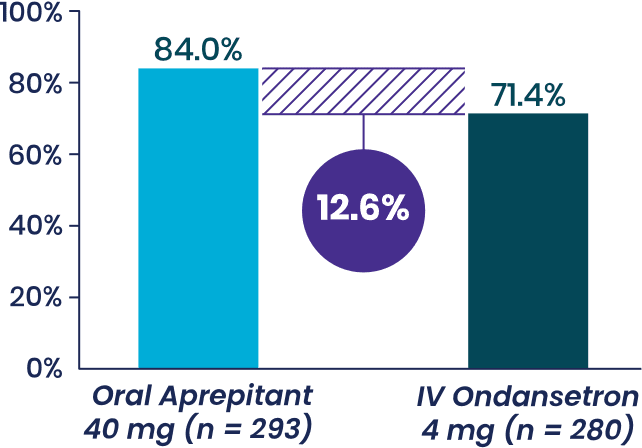
Aprepitant demonstrated
superiority to IV ondansetron (
Study 2 Primary Endpoint
% patients with with no vomiting,
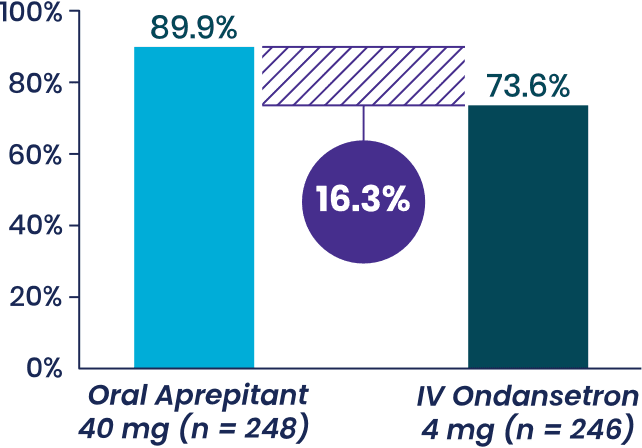
Aprepitant demonstrated superiority to
IV ondansetron (
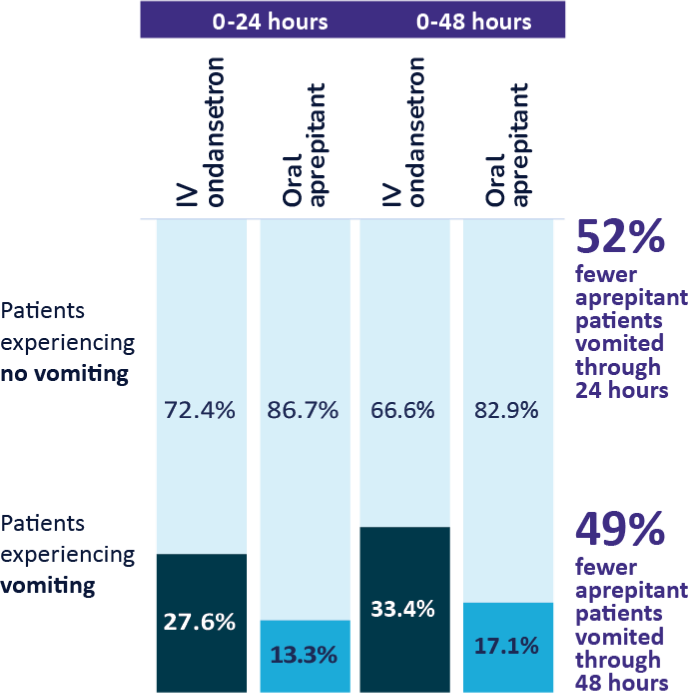
Superior Vomiting Prevention Through 48 hours
1-3
Study 1 Secondary Endpoint
% patients with with no vomiting,
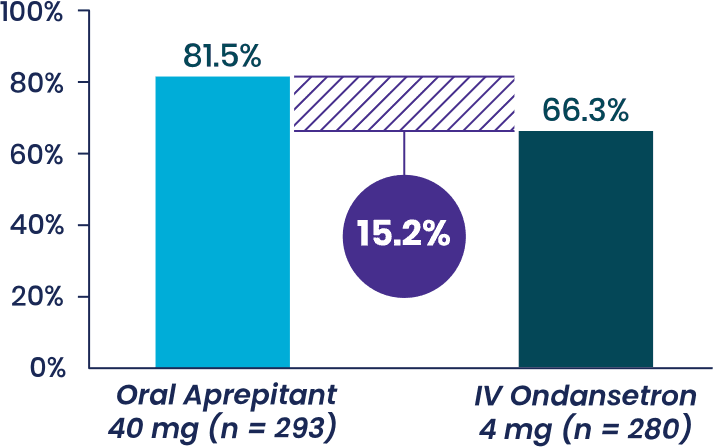
- Aprepitant demonstrated superiority to
IV ondansetron (
P < .001b ) - Half as many patients vomited when treated with oral aprepitant compared to IV ondansetron
Study 2 Secondary Endpoint
% patients with with no vomiting,
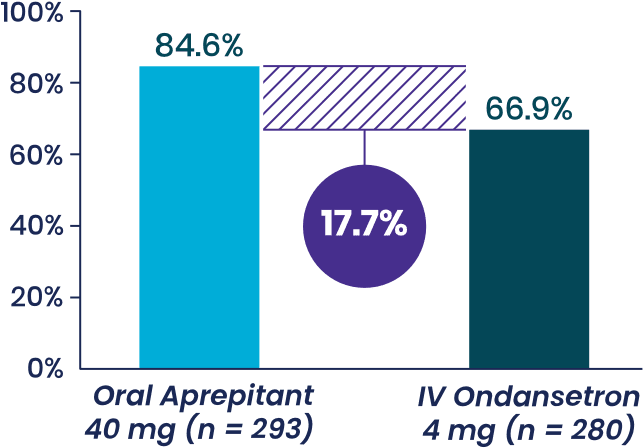
- Aprepitant demonstrated superiority to IV ondansetron (P < .001c)
- In an analysis adjusting for rescue therapy, patients receiving oral aprepitant experienced 75% fewer episodes of vomiting, on average, compared to those taking IV ondansetron
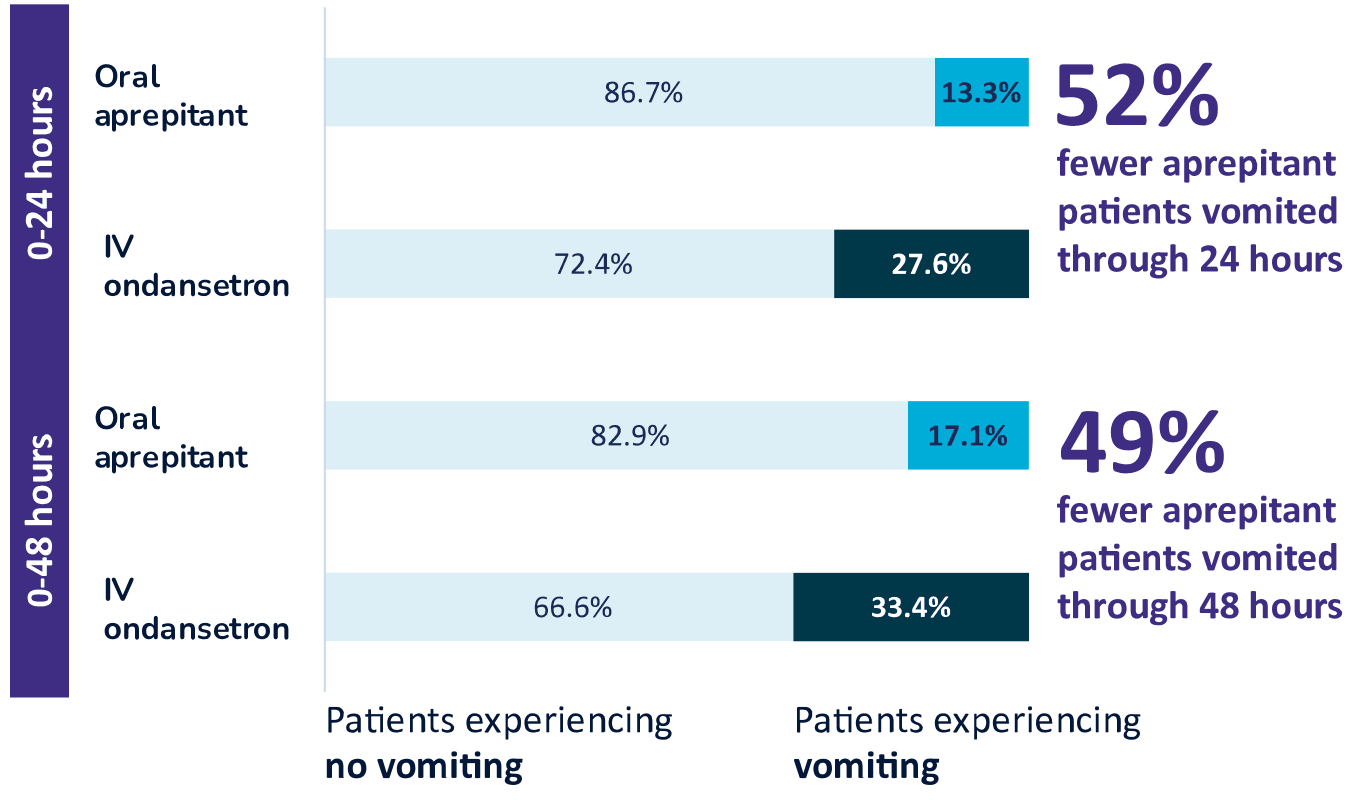
Pooled Analysis of Phase 3 Clinical Studies1-3,d
Approximately 50% fewer patients vomited in the first 24 and 48 hours after receiving aprepitant
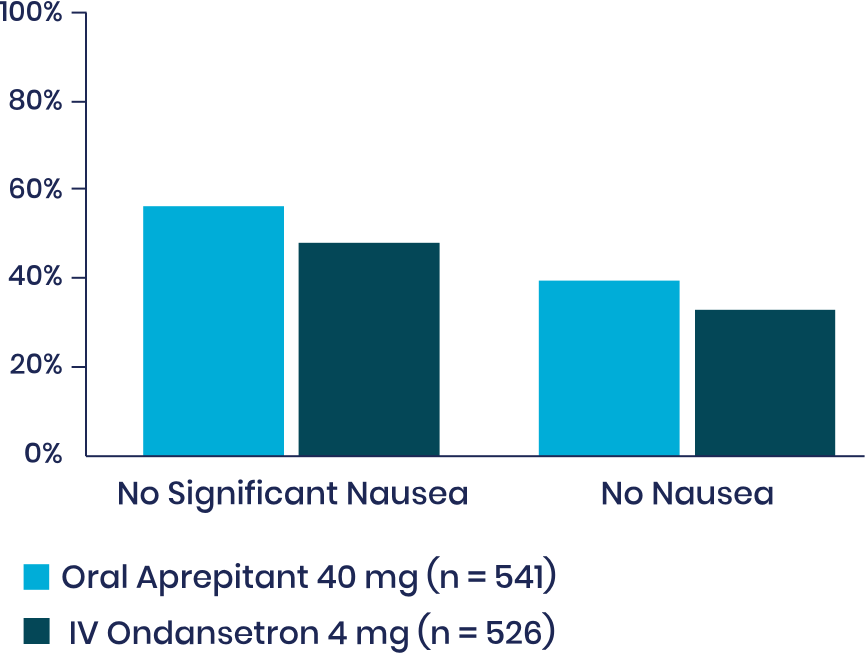
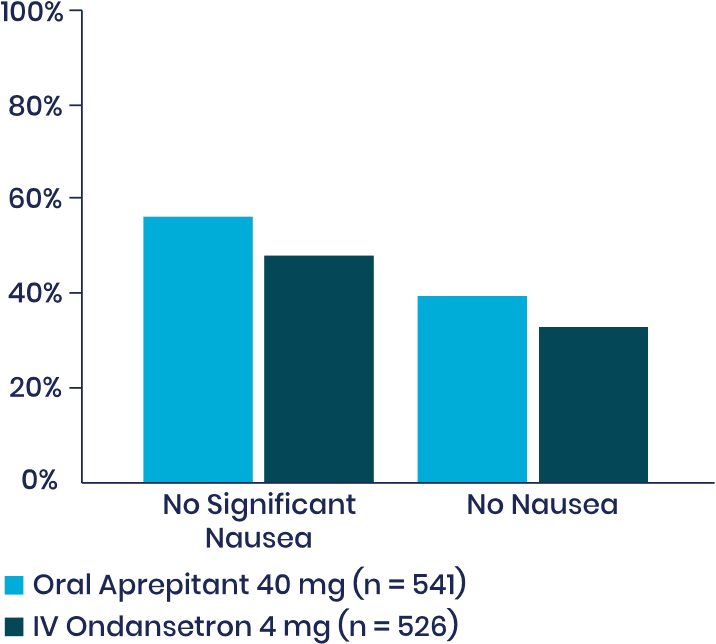
Post-Hoc Analysis4
Nausea: Aprepitant Versus IV ondansetron
Pooled data from the 2 randomized, active-controlled clinical trials of oral aprepitant supporting the approval of APONVIE in the US were analyzed to further explore the potential efficacy profile of aprepitant versus IV ondansetron in terms of nausea and use of rescue therapy in the first 24 hours following surgery.
Note: Nausea was rated on a verbal rating scale of 0 to 10, with a score of 0 meaning no nausea and a score of 10 meaning the worst nausea ever. No significant nausea was defined as a peak VRS score ≤4 in the first 24 hours. Rescue therapy was available if the patient requested it, if the patient had >1 episode of vomiting or retching, or if the patient had nausea lasting longer than 15 minutes. When increasing statistical power with this combined sample, nominal P < .035 for each comparison in favor of aprepitant 40 mg—warranting a further, prospective study. (Two-tail P of the odds ratio vs IV ondansetron 4 mg for each exploratory endpoint.)
aOR: estimated odds ratio for aprepitant versus IV ondansetron. A value of >1 favors aprepitant over IV ondansetron. LB: lower bound. Superiority: if LB > 1.
bP value of 2-tail test at the .05 significance level.
cUnadjusted P value.
dDescriptive statistics pooled across 2 randomized, double-blind trials of oral aprepitant 40 mg (0-24 hours: n = 541, 0-48 hours: n = 539) versus active-control ondansetron IV 4 mg (0-24 hours: n = 526, 0-48 hours: n = 525) for the prevention of PONV following open abdominal surgery under general anesthesia.
To learn more about APONVIE from a peer, connect with Heron to set up a clinical consult.
Connect with HeronReferences: 1. APONVIE [package
insert]. San Diego, CA: Heron Therapeutics Inc; 2022. 2. Diemunsch
P, Gan TJ, Philip BK, et al. Single-dose aprepitant vs ondansetron for the
prevention of postoperative nausea and vomiting: a randomized, double-blind Phase
III trial in patients undergoing open abdominal surgery. Brit J Anaesth.
2007;99(2):202-211. doi:10.1093/bja/aem133.
Important Safety Information!
Contraindications
APONVIE is contraindicated in patients with a history of hypersensitivity to aprepitant or any component of the product, and in patients taking pimozide. Increased pimozide levels may cause serious or life-threatening reactions, such as QT prolongation.
Warnings and Precautions
Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis, during or soon after administration of aprepitant have occurred. Symptoms including dyspnea, eye swelling, flushing, pruritus, and wheezing have been reported. Monitor patients during and after administration. If hypersensitivity reactions occur, administer appropriate medical therapy. Do not administer APONVIE in patients who experienced these symptoms with previous use of aprepitant.
Clinically Significant CYP3A4 Drug Interactions: Aprepitant is a substrate, weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Use of pimozide, a CYP3A4 substrate, with APONVIE is contraindicated. Use of APONVIE with strong CYP3A4 inhibitors (eg, ketoconazole) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to APONVIE. Use of APONVIE with strong CYP3A4 inducers (eg, rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of APONVIE.
Decrease in INR with Concomitant Warfarin: Use of aprepitant with
warfarin, a CYP2C9 substrate, may result in a clinically significant
decrease in the International Normalized Ratio (INR) of prothrombin time.
Monitor the INR in patients on chronic warfarin therapy in the 2-week period
particularly at 7 to
Risk of Reduced Efficacy of Hormonal Contraceptives: The efficacy of
hormonal contraceptives may be reduced for
Use in Specific Populations
Avoid use of APONVIE in pregnant women as alcohol is an inactive ingredient in APONVIE. There is no safe level of alcohol exposure in pregnancy.
Adverse Reactions
Most common adverse reactions (incidence ≥3%) for APONVIE are constipation, fatigue, and headache and for oral aprepitant are constipation and hypotension.
Report side effects to Heron at
Indication
APONVIE is a substance P/neurokinin-1 (NK1) receptor antagonist, indicated for the prevention of postoperative nausea and vomiting (PONV) in adults.
Limitations of Use: APONVIE has not been studied for treatment of established nausea and vomiting.
Please see full Prescribing Information.
Connect with Heron!
How could you incorporate APONVIE as the foundation of your institution's PONV management strategy? Connect with us to find out.
Fields marked with an asterisk (*) are required.
To report an adverse event or product complaint:
844-HERON11 (844-437-6611)
MedInfo@HeronTx.com
For general information:
Investors and Media:
For all other inquiries, please visit the Heron Therapeutics corporate website.
Go to Heron Website