APONVIE as the Foundation of a Multimodal Regimen for Patients at Moderate-to-High Risk of PONV
Guidelines for Multimodal Treatment
According to the newest version of the Society for Ambulatory Anesthesiology’s consensus guidelines on the management of PONV, multimodal prophylaxis should be considered for patients with one or more risk factors.1
As an NK1 antagonist (a guideline-recommended class of PONV medications), APONVIE has little or no affinity for serotonin (5-HT3), dopamine, and corticosteroid receptors, the targets of other therapies for PONV, which makes it a good option for use as a first-line therapy in a multimodal approach for patients at moderate-to-high risk.1,2
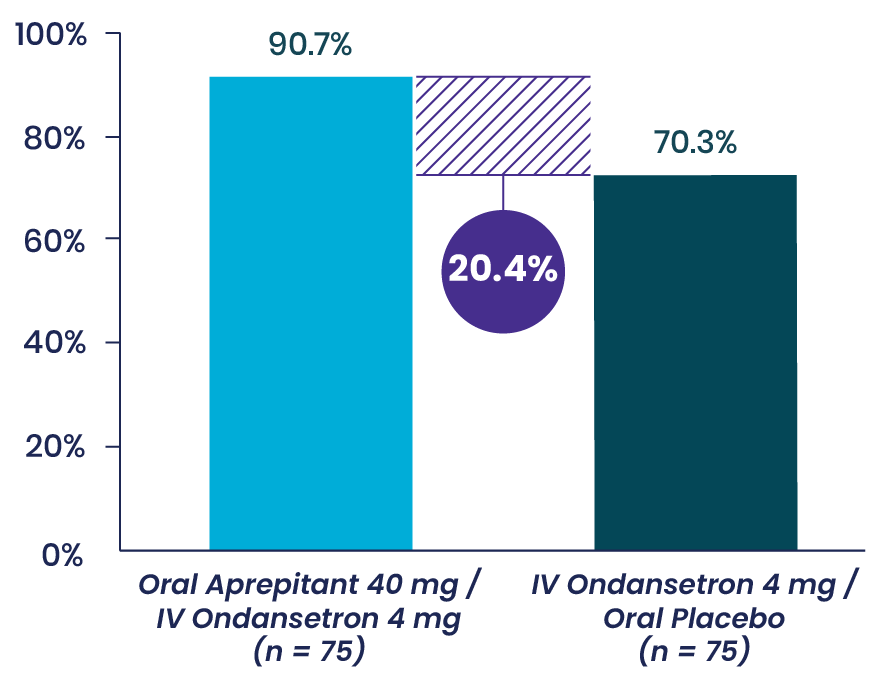
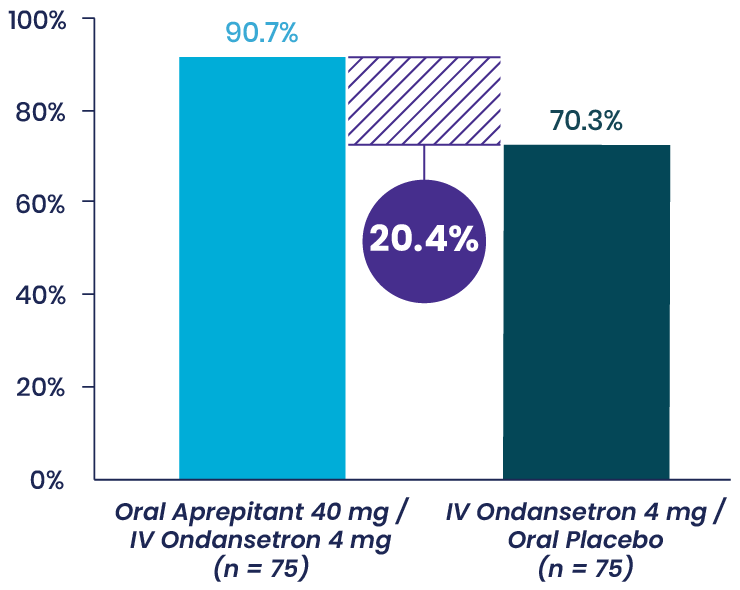
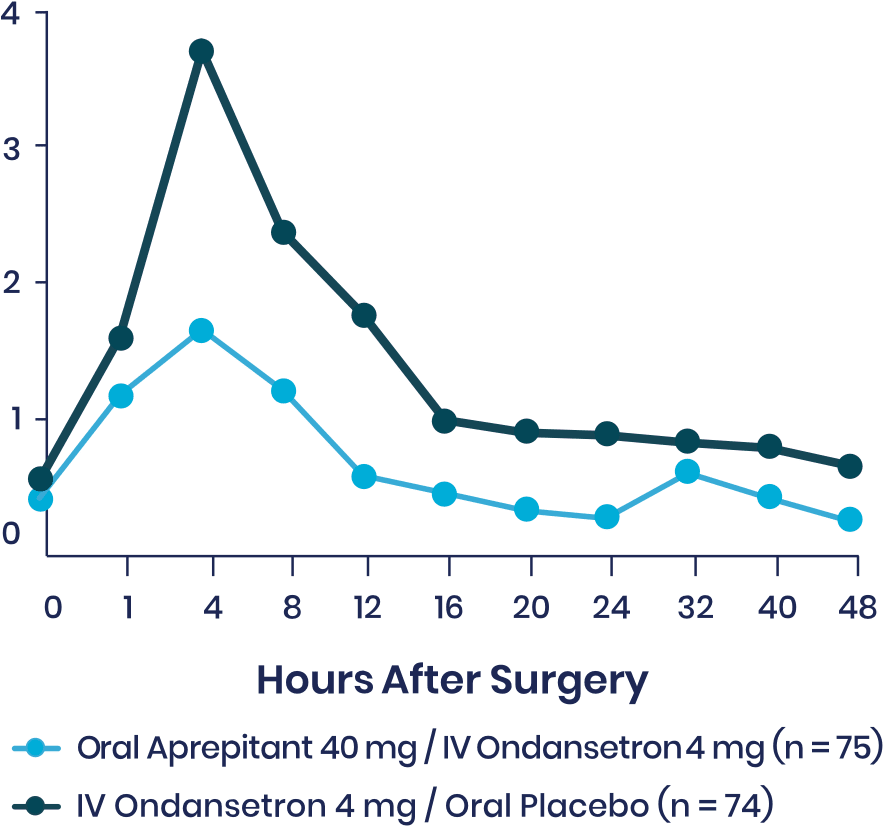
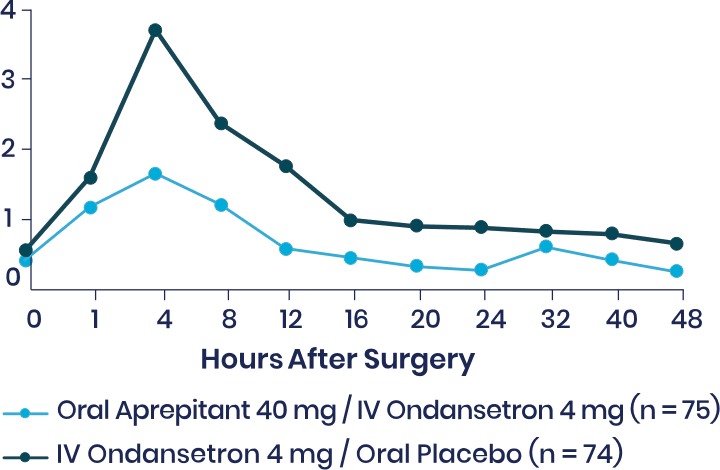
Vallejo et al: Prospective Study Shows Aprepitant as Part of a Multimodal Approach Is Highly Effective for Moderate-to-High-Risk Plastic Surgery Patients3
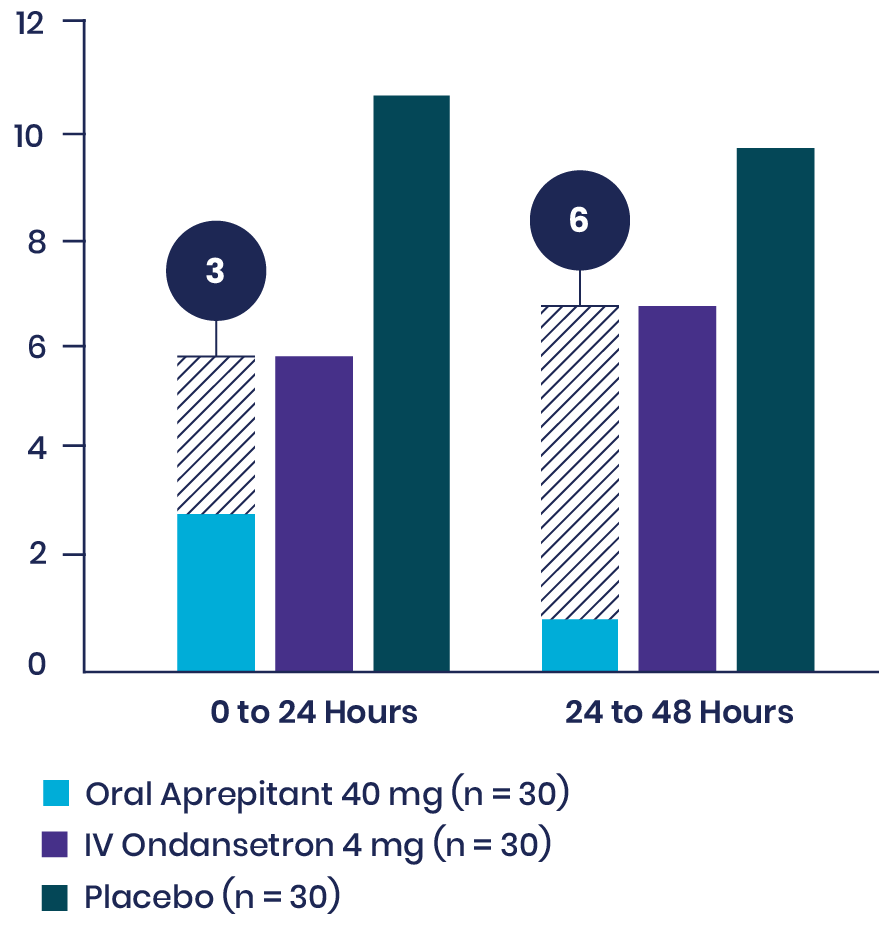
This prospective, double-blinded, randomized 2-arm study evaluated the occurrence of vomiting and the severity of nausea in the 48 hours following surgery in 150 ambulatory plastic surgery patients undergoing general anesthesia with 2 or more risk factors for PONV. Patients received either aprepitant plus IV ondansetron or IV ondansetron and placebo.
Primary Endpoint
Secondary Endpoint
Note: Nausea was rated on a verbal rating scale of 0 to 10, with a score of 0 meaning no nausea and a score of 10 meaning the worst nausea ever.
aMean differences in VRS scores over 48 hours tested simultaneously with a multivariate analysis of variance.
bUnadjusted P value.
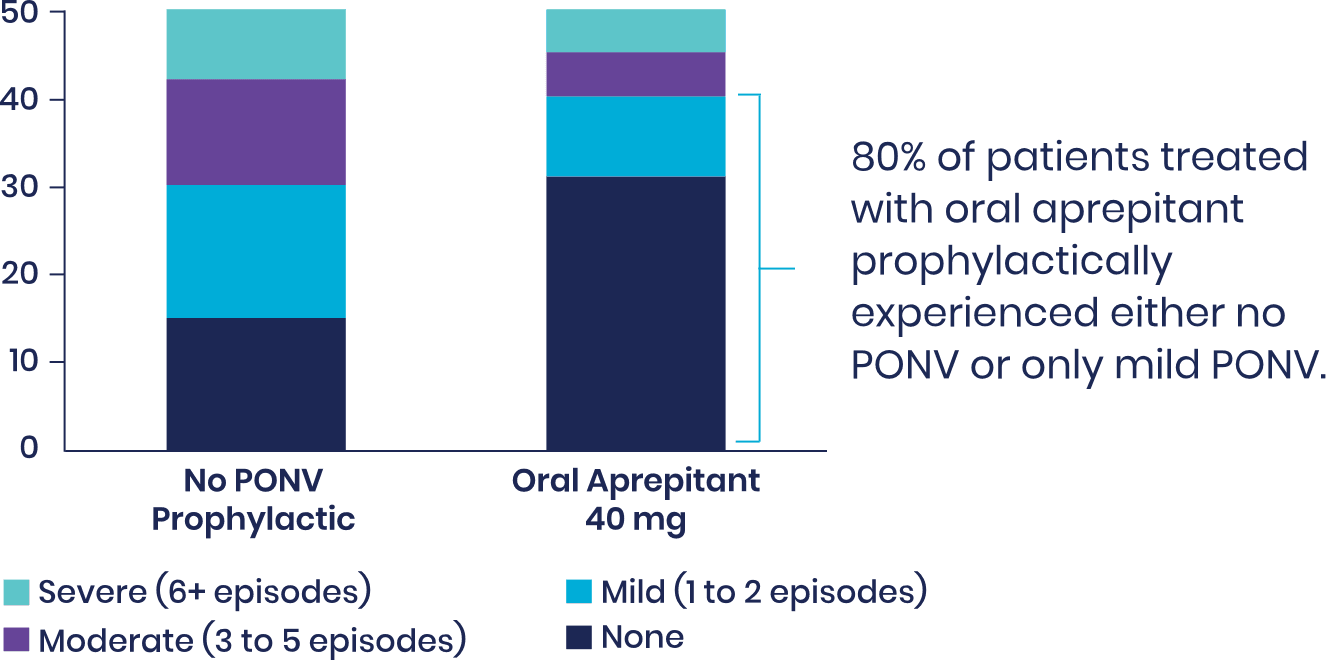
View studyDilorio et al: Retrospective Chart Review Showed that Total Knee Arthroplasty and Total Hip Arthroplasty Patients Receiving Aprepitant Experienced Reduced Severity of PONV4
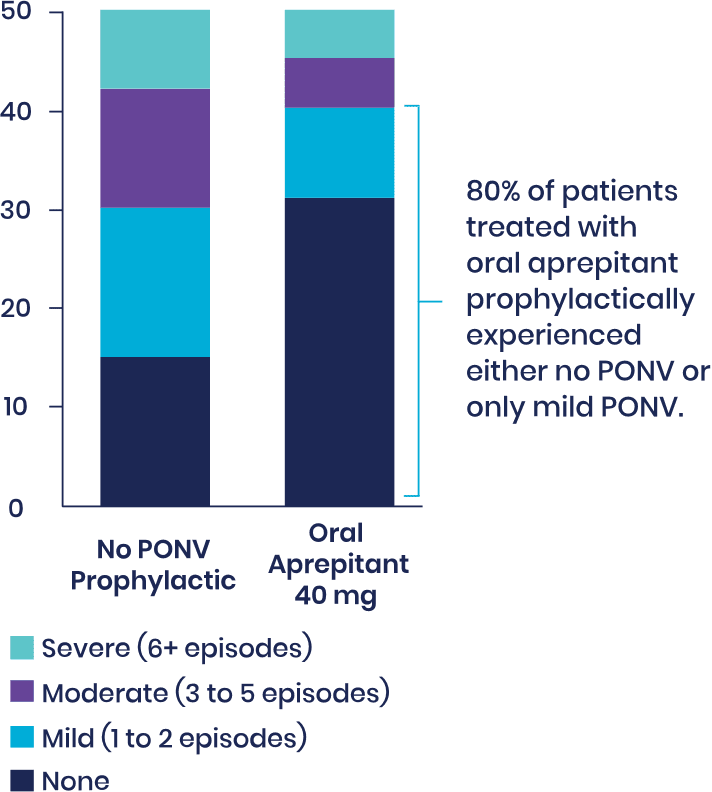
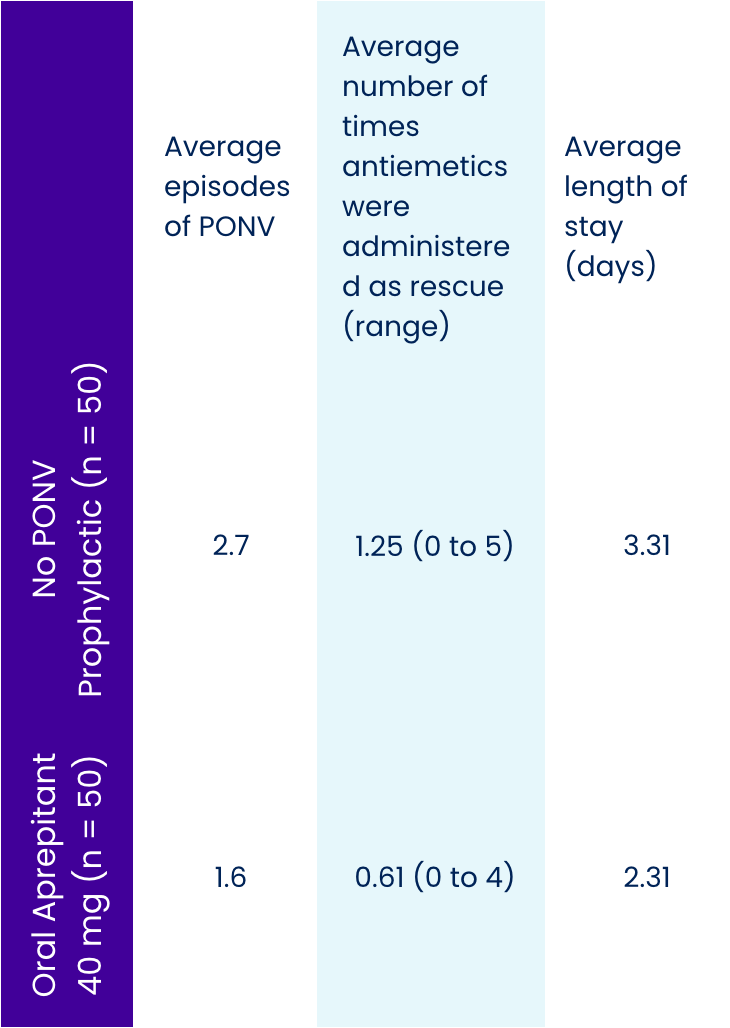
Fifty patients undergoing total hip arthroplasty or total knee arthroplasty who received a preoperative dose of oral aprepitant were matched to 50 patients who received no PONV prophylactic. Patients in both groups were treated postoperatively with IV ondansetron 4 mg only as rescue medication if PONV occurred. Patients' charts were retrospectively reviewed. Instances of PONV were noted for the entire length of hospital stay.
Severity of PONV
Number of patients
Key Metrics of PONV Reduced
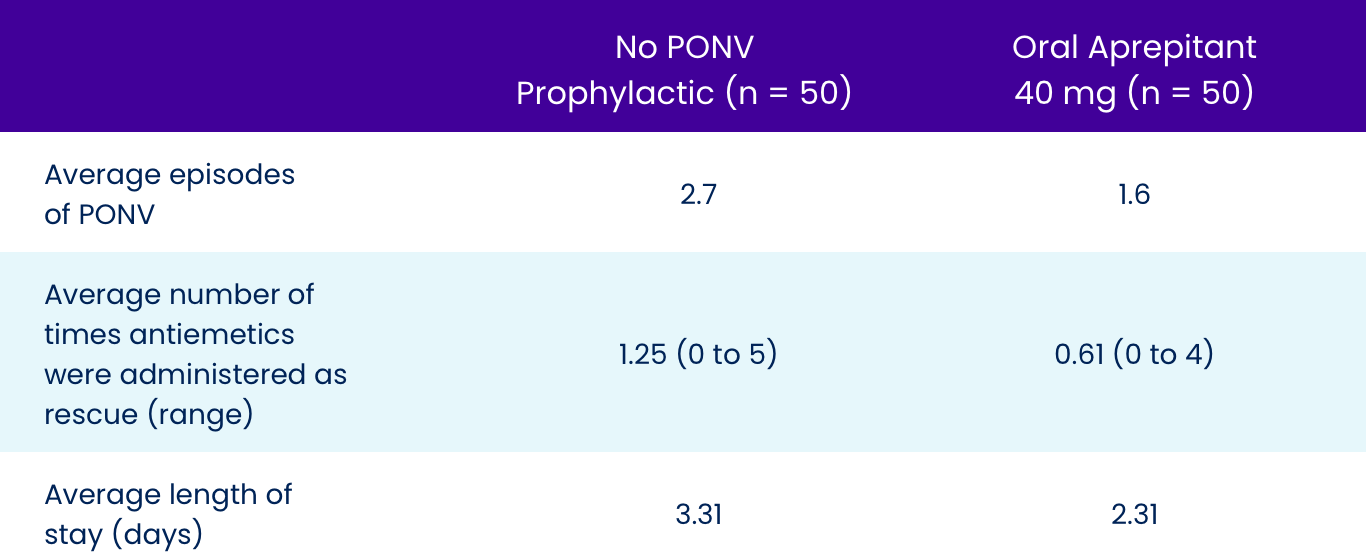
On average, patients receiving oral aprepitant had approximately 40% fewer episodes of PONV, required less than half the amount of rescue medication, and were ready for discharge a day sooner.
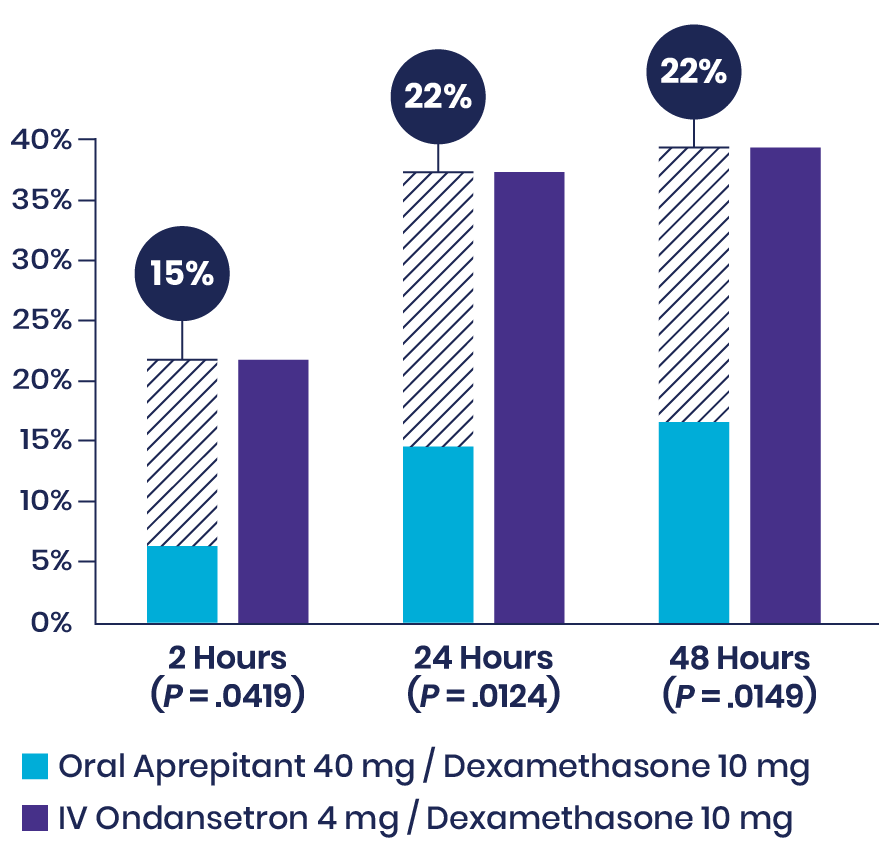
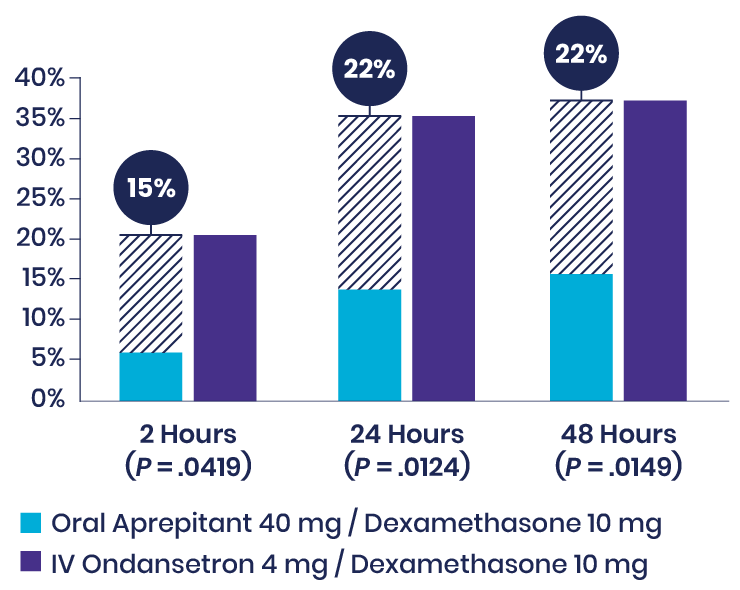
Habib et al: Prospective Study Showed That Aprepitant Reduced the Incidence of Vomiting in Craniotomy Patients5
In this prospective, double-blind, randomized study of 104 patients undergoing craniotomy under general anesthesia, patients received dexamethasone as well as either oral aprepitant or IV ondansetron. Though the complete response was similar between treatment groups, using aprepitant in addition to dexamethasone significantly reduced the incidence of vomiting compared to IV ondansetron at multiple timepoints.
Incidence of Vomiting
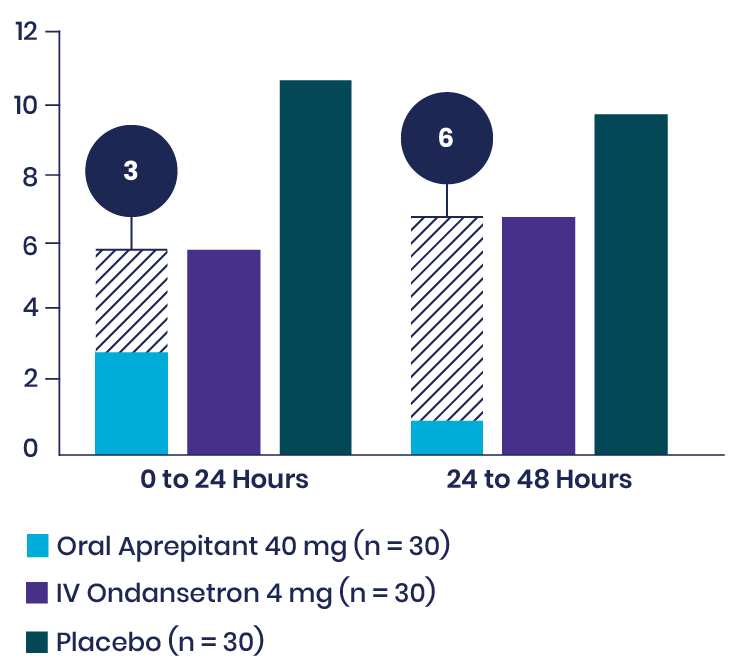
View studyKaur et al: Prospective Study Showed Aprepitant Demonstrated a Longer-Acting Effect than IV ondansetron in Laparoscopic Cholecystectomy6
In a randomized, double-blind, prospective trial, 90 patients undergoing laparoscopic cholecystectomy were treated with either oral aprepitant 40 mg, IV ondansetron 4 mg, or placebo. Though nausea scores across both treatment groups were similar, and the difference in the number of episodes of vomiting was not statistically significant in the first 24 hours, fewer episodes of vomiting were reported in the 24- to 48-hour time period in patients treated with aprepitant than in those treated with IV ondansetron.
Episodes of Vomiting
View studyTrimas et al: Retrospective Analysis Shows Aprepitant Reduced Postoperative Nausea in Facial Plastic Surgery7
In a retrospective analysis of 172 consecutive patients undergoing facial plastic surgery with general anesthesia, patients were treated with IV ondansetron and dexamethasone. In addition, 56 patients also received aprepitant. The addition of aprepitant reduced the likelihood of postoperative nausea compared to IV ondansetron and dexamethasone.
98% of patients receiving aprepitant in addition to IV ondansetron and dexamethasone experienced no PONV
84% of patients receiving only IV ondansetron and dexamethasone experienced no PONV
Hartrick et al: Open-Label Study Showed Aprepitant Outperformed Multimodal PONV Therapies in TKA8
In a sequential, open-label, matched case control study, 24 patients underwent
total knee arthroplasty with extended-release epidural morphine. One group of
patients received ondansetron and dexamethasone, combined with either
metoclopramide, diphenhydramine, or prochlorperazine, while the other received
only oral aprepitant preoperatively. Incidence of PONV was then compared between
the two groups. In this study, patients were considered positive for PONV if
they reported any incidence of nausea or vomiting. Aprepitant used as a
single agent significantly reduced the incidence of PONV compared to a
multimodal antiemetic regimen (
75% of patients who received aprepitant experienced no PONV
25% of patients who received the multimodal therapy experienced no PONV
Resources
PONV Guidelines: Gan, et al.
Vallejo, et al.
Dilorio, et al.
Habib, et al.
Kaur, et al.
Trimas, et al.
Hartrick, et al.
References: 1. Gan TJ, Belani KG,
Bergese S, et al. Fourth consensus guidelines for the management of postoperative
nausea and vomiting. Anesth Analg. 2020;131(2):411-448.
doi:10.1213/ane.0000000000004833.
Important Safety Information!
Contraindications
APONVIE is contraindicated in patients with a history of hypersensitivity to aprepitant or any component of the product, and in patients taking pimozide. Increased pimozide levels may cause serious or life-threatening reactions, such as QT prolongation.
Warnings and Precautions
Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis, during or soon after administration of aprepitant have occurred. Symptoms including dyspnea, eye swelling, flushing, pruritus, and wheezing have been reported. Monitor patients during and after administration. If hypersensitivity reactions occur, administer appropriate medical therapy. Do not administer APONVIE in patients who experienced these symptoms with previous use of aprepitant.
Clinically Significant CYP3A4 Drug Interactions: Aprepitant is a substrate, weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Use of pimozide, a CYP3A4 substrate, with APONVIE is contraindicated. Use of APONVIE with strong CYP3A4 inhibitors (eg, ketoconazole) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to APONVIE. Use of APONVIE with strong CYP3A4 inducers (eg, rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of APONVIE.
Decrease in INR with Concomitant Warfarin: Use of aprepitant with
warfarin, a CYP2C9 substrate, may result in a clinically significant
decrease in the International Normalized Ratio (INR) of prothrombin time.
Monitor the INR in patients on chronic warfarin therapy in the 2-week period
particularly at 7 to
Risk of Reduced Efficacy of Hormonal Contraceptives: The efficacy of
hormonal contraceptives may be reduced for
Use in Specific Populations
Avoid use of APONVIE in pregnant women as alcohol is an inactive ingredient in APONVIE. There is no safe level of alcohol exposure in pregnancy.
Adverse Reactions
Most common adverse reactions (incidence ≥3%) for APONVIE are constipation, fatigue, and headache and for oral aprepitant are constipation and hypotension.
Report side effects to Heron at
Indication
APONVIE is a substance P/neurokinin-1 (NK1) receptor antagonist, indicated for the prevention of postoperative nausea and vomiting (PONV) in adults.
Limitations of Use: APONVIE has not been studied for treatment of established nausea and vomiting.
Please see full Prescribing Information.
Connect with Heron!
How could you incorporate APONVIE as the foundation of your institution's PONV management strategy? Connect with us to find out.
Fields marked with an asterisk (*) are required.
To report an adverse event or product complaint:
844-HERON11 (844-437-6611)
MedInfo@HeronTx.com
For general information:
Investors and Media:
For all other inquiries, please visit the Heron Therapeutics corporate website.
Go to Heron Website