The Safety Profile of APONVIE
Similar Safety Profile to That of IV Ondansetron
Aprepitant has been used for PONV since 2006.1
In 2 studies of more than 560 patients undergoing general anesthesia, there were no significant differences in the incidence of adverse events between oral aprepitant and IV ondansetron.2-4
Aprepitant is not associated with QT prolongation, urinary retention, blurred vision, cognitive issues including sedation, or instances of serotonin syndrome.2
Aprepitant is well tolerated, with an established safety profile.2
IV aprepitant has been administered, even at a higher dose (
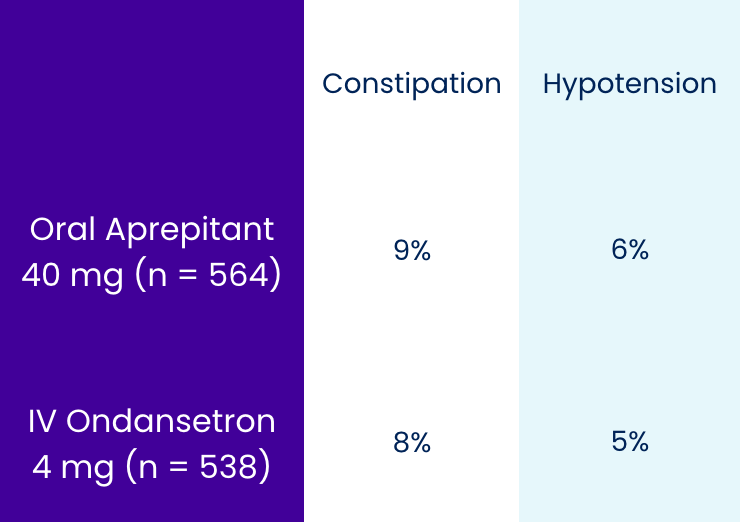
Pooled Analysis: Adverse Reactions2

Events included adverse reactions with an incidence ≥3% and at a greater incidence than IV ondansetron.
To learn more about APONVIE from a peer, connect with Heron to set up a clinical consult.
Connect with HeronReferences: 1. Korvick J; Center for
Drug Evaluation and Research. Supplemental NDA approval letter: NDA 21-549/S-010
(Emend). Rockville, MD: US Food and Drug Administration; June 30, 2006.
https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2006/021549s010LTR.pdf.
Accessed March 14, 2022.
Important Safety Information!
Contraindications
APONVIE is contraindicated in patients with a history of hypersensitivity to aprepitant or any component of the product, and in patients taking pimozide. Increased pimozide levels may cause serious or life-threatening reactions, such as QT prolongation.
Warnings and Precautions
Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis, during or soon after administration of aprepitant have occurred. Symptoms including dyspnea, eye swelling, flushing, pruritus, and wheezing have been reported. Monitor patients during and after administration. If hypersensitivity reactions occur, administer appropriate medical therapy. Do not administer APONVIE in patients who experienced these symptoms with previous use of aprepitant.
Clinically Significant CYP3A4 Drug Interactions: Aprepitant is a substrate, weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Use of pimozide, a CYP3A4 substrate, with APONVIE is contraindicated. Use of APONVIE with strong CYP3A4 inhibitors (eg, ketoconazole) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to APONVIE. Use of APONVIE with strong CYP3A4 inducers (eg, rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of APONVIE.
Decrease in INR with Concomitant Warfarin: Use of aprepitant with
warfarin, a CYP2C9 substrate, may result in a clinically significant
decrease in the International Normalized Ratio (INR) of prothrombin time.
Monitor the INR in patients on chronic warfarin therapy in the 2-week period
particularly at 7 to
Risk of Reduced Efficacy of Hormonal Contraceptives: The efficacy of
hormonal contraceptives may be reduced for
Use in Specific Populations
Avoid use of APONVIE in pregnant women as alcohol is an inactive ingredient in APONVIE. There is no safe level of alcohol exposure in pregnancy.
Adverse Reactions
Most common adverse reactions (incidence ≥3%) for APONVIE are constipation, fatigue, and headache and for oral aprepitant are constipation and hypotension.
Report side effects to Heron at
Indication
APONVIE is a substance P/neurokinin-1 (NK1) receptor antagonist, indicated for the prevention of postoperative nausea and vomiting (PONV) in adults.
Limitations of Use: APONVIE has not been studied for treatment of established nausea and vomiting.
Please see full Prescribing Information.
Connect with Heron!
How could you incorporate APONVIE as the foundation of your institution's PONV management strategy? Connect with us to find out.
Fields marked with an asterisk (*) are required.
To report an adverse event or product complaint:
844-HERON11 (844-437-6611)
MedInfo@HeronTx.com
For general information:
Investors and Media:
For all other inquiries, please visit the Heron Therapeutics corporate website.
Go to Heron Website