Proven Bioequivalence to Oral Aprepitant
Proven Bioequivalence to Oral Aprepitant 40 mg in a Phase 1 Clinical Study
This study showed that APONVIE behaves predictably in the human body and shows bioequivalence given overall exposure to oral aprepitant.1
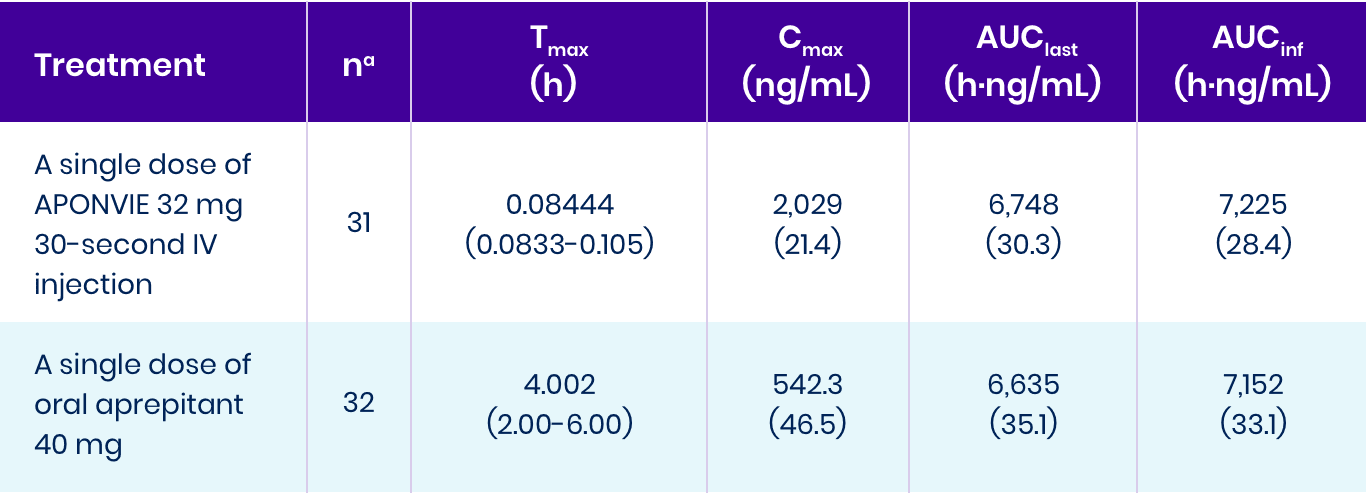
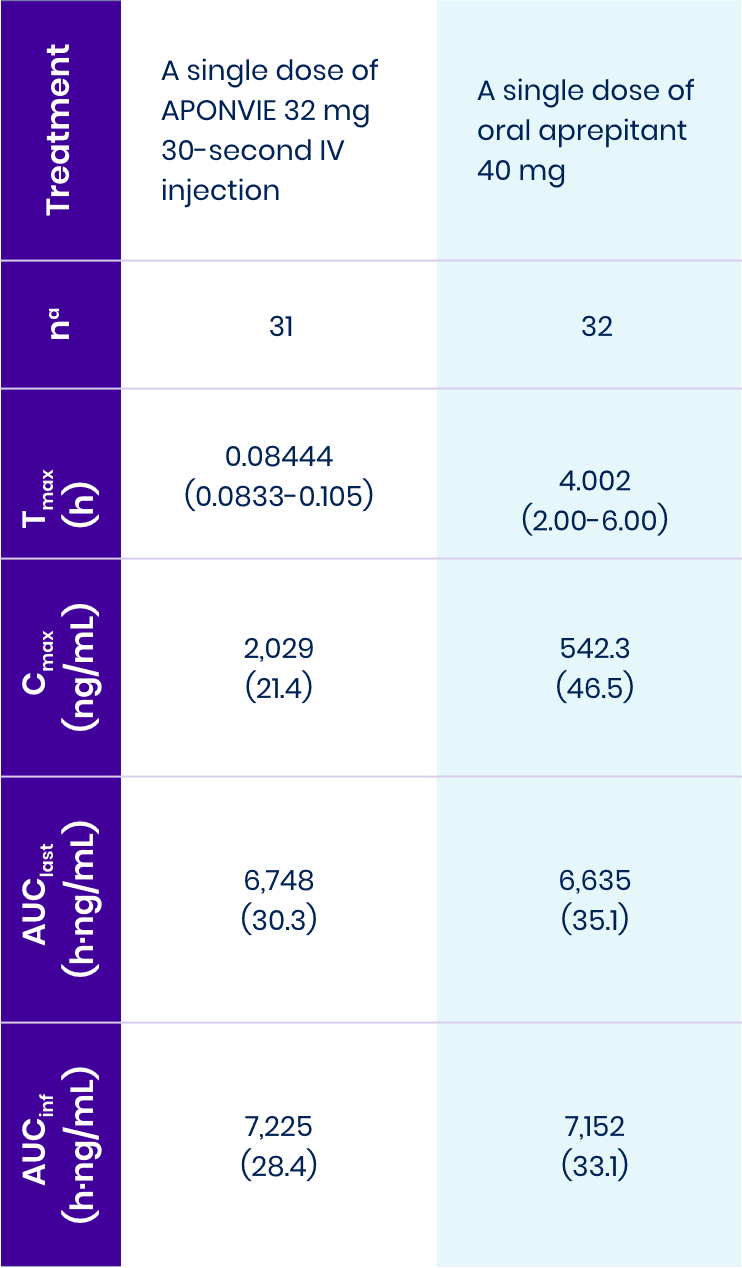
aOne subject in Study 111 was excluded from the APONVIE treatment due to an atypical PK profile for APONVIE. AUCinf was not reportable for 2 subjects (one from each treatment group) because they failed to meet the inclusion criteria.
AUC0-24h: area under the concentration-time curve from 0 to 24 hours post dose.
AUCinf: area under the concentration-time curve extrapolated to infinity.
AUClast: area under the concentration-time curve to the time of the last quantifiable concentration.
Cmax: maximum concentration.
Tmax: time of occurrence of maximum concentration.
View efficacy data for oral aprepitant »Onset of Action
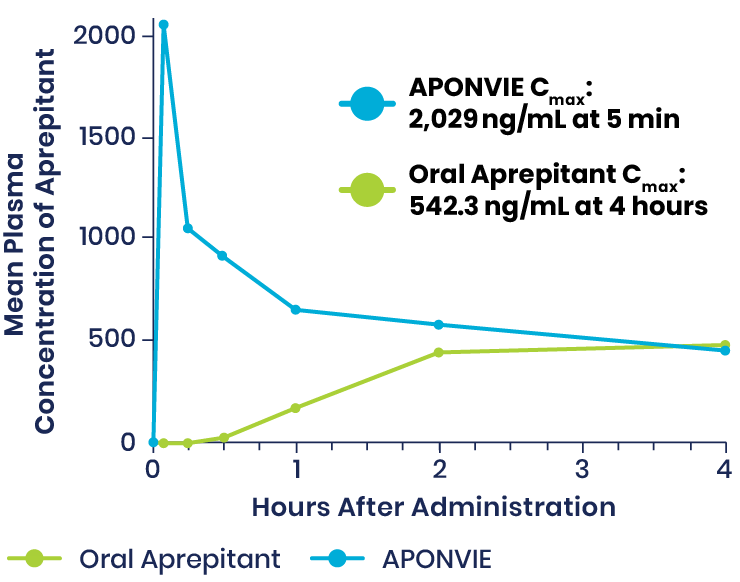
APONVIE is delivered via a single IV push. Therapeutic plasma concentrations associated with ≥97% receptor occupancy in the brain are achieved within 5 minutes for APONVIE—unlike oral aprepitant, which was taken 1 to 3 hours prior to induction of general anesthesia in clinical trials and does not reach maximum concentration until 3 hours after administration.2-5,a
At 48 hours, therapeutic plasma concentrations associated with NK1 receptor occupancy is estimated to be maintained at >90%.1,3,a
aThe relationship between receptor occupancy and efficacy has not been established.
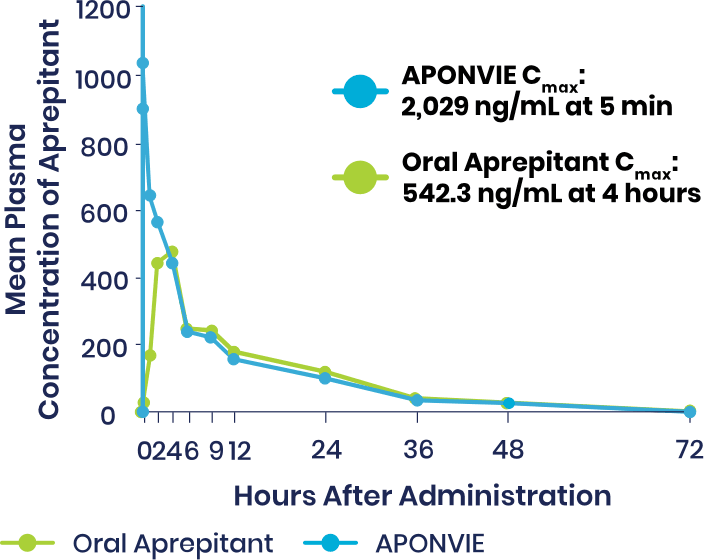
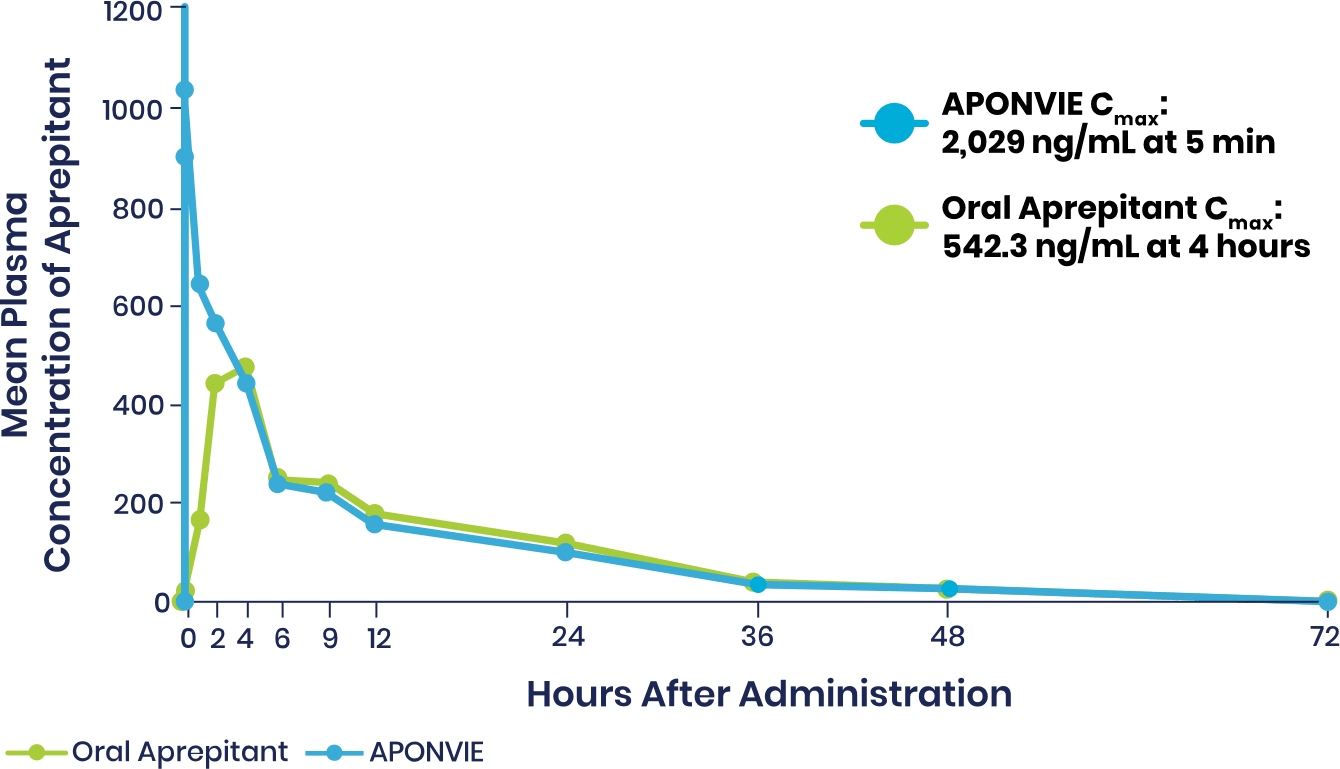
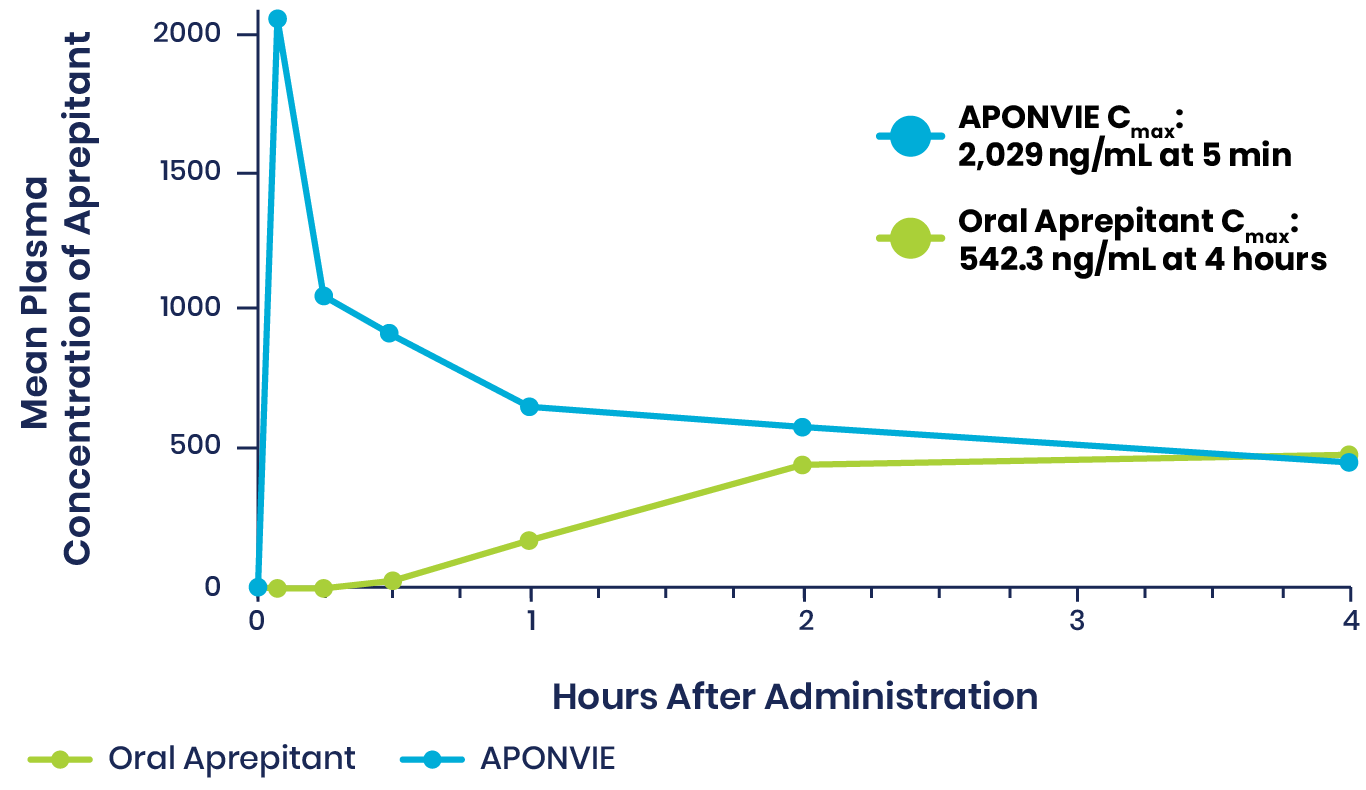
Plasma Concentrations of APONVIE Versus Oral Aprepitant
Plasma concentrations of aprepitant with APONVIE were higher than those of the oral formulation for approximately 3 hours. By the 4-hour timepoint, the plasma concentrations for the 2 formulations converged and remained similar.1
IV administration allows the drug to enter directly into systemic circulation without the delay associated with absorption processes. This results in 100% bioavailability, making it the best way to deliver a drug rapidly and accurately, and bypassing first-pass metabolism.6
Resources
References: 1. Data on file. Study
HTX-019-111. San Diego, CA: Heron Therapeutics Inc; 2021.
Important Safety Information!
Contraindications
APONVIE is contraindicated in patients with a history of hypersensitivity to aprepitant or any component of the product, and in patients taking pimozide. Increased pimozide levels may cause serious or life-threatening reactions, such as QT prolongation.
Warnings and Precautions
Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis, during or soon after administration of aprepitant have occurred. Symptoms including dyspnea, eye swelling, flushing, pruritus, and wheezing have been reported. Monitor patients during and after administration. If hypersensitivity reactions occur, administer appropriate medical therapy. Do not administer APONVIE in patients who experienced these symptoms with previous use of aprepitant.
Clinically Significant CYP3A4 Drug Interactions: Aprepitant is a substrate, weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Use of pimozide, a CYP3A4 substrate, with APONVIE is contraindicated. Use of APONVIE with strong CYP3A4 inhibitors (eg, ketoconazole) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to APONVIE. Use of APONVIE with strong CYP3A4 inducers (eg, rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of APONVIE.
Decrease in INR with Concomitant Warfarin: Use of aprepitant with
warfarin, a CYP2C9 substrate, may result in a clinically significant
decrease in the International Normalized Ratio (INR) of prothrombin time.
Monitor the INR in patients on chronic warfarin therapy in the 2-week period
particularly at 7 to
Risk of Reduced Efficacy of Hormonal Contraceptives: The efficacy of
hormonal contraceptives may be reduced for
Use in Specific Populations
Avoid use of APONVIE in pregnant women as alcohol is an inactive ingredient in APONVIE. There is no safe level of alcohol exposure in pregnancy.
Adverse Reactions
Most common adverse reactions (incidence ≥3%) for APONVIE are constipation, fatigue, and headache and for oral aprepitant are constipation and hypotension.
Report side effects to Heron at
Indication
APONVIE is a substance P/neurokinin-1 (NK1) receptor antagonist, indicated for the prevention of postoperative nausea and vomiting (PONV) in adults.
Limitations of Use: APONVIE has not been studied for treatment of established nausea and vomiting.
Please see full Prescribing Information.
Connect with Heron!
How could you incorporate APONVIE as the foundation of your institution's PONV management strategy? Connect with us to find out.
Fields marked with an asterisk (*) are required.
To report an adverse event or product complaint:
844-HERON11 (844-437-6611)
MedInfo@HeronTx.com
For general information:
Investors and Media:
For all other inquiries, please visit the Heron Therapeutics corporate website.
Go to Heron Website